Running on Liquid: The Surprising Science Behind Oobleck
Written on
Chapter 1: Understanding Liquid Dynamics
Can we actually run on a liquid? The conventional belief holds that solids can bear weight, allowing us to walk on them, while liquids cannot. This stems from the behavior of atoms in solids, which resemble springs. When force is exerted on a spring, it pushes back with equal force. This principle can be illustrated by jumping on a mattress or trampoline—the harder you jump, the more force is exerted back upward.
Understanding the atomic structure of solids is key to this concept. When stress (force per unit area) is applied to a solid, its atomic components shift like springs (strain), pushing back against the force. This interaction is vital for our ability to walk; for example, when stepping on concrete, you slightly compress its atoms, generating a supportive force beneath you.
For liquids, however, any stress results in the atoms flowing, causing the opposite effect. The more force you apply, the more the liquid moves. If you dive into a pool, you sink deeper than if you simply wade in. Nevertheless, many everyday materials challenge this traditional view of solids versus liquids. One notable example is cornstarch.
Section 1.1: The Unique Properties of Oobleck
When cornstarch is mixed with water, it creates a thick, soupy mixture. As the proportion of cornstarch increases, the mixture's thickness changes. This concoction, known as Oobleck, behaves differently under various conditions. For instance, if you attempt to slowly stir a spoon through it, the spoon glides through easily, similar to how it would through honey. However, if you try to stir quickly, Oobleck resists and can even appear to solidify under stress.

What causes this unique behavior? To understand, we must first define viscosity, which describes a liquid's response to deformation. Mathematically, viscosity is the stress required to deform a liquid at a specific rate, related to how quickly it spreads.
Subsection 1.1.1: The Science of Shear Thickening
Oobleck exhibits variable viscosity depending on the force applied. This phenomenon occurs because cornstarch consists of tiny grains. When pressure is applied slowly, the grains can move past one another with little resistance. Conversely, applying a significant force causes these grains to collide and get stuck, leading to increased viscosity.
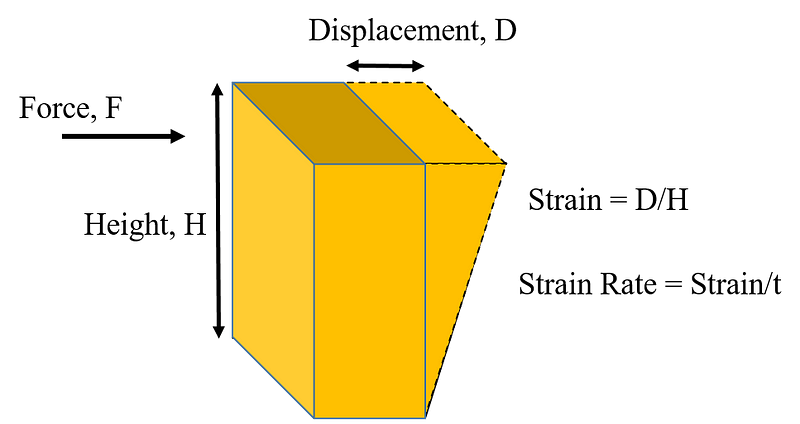
The phenomenon of increasing viscosity with applied stress is termed shear thickening. While this explains Oobleck’s response to force, it doesn’t fully clarify why it can support the weight of a person running or jumping on it. A study conducted in 2012 by physicists Scott Waitukaitis and Heinrich Jaeger revealed that when you jump into a cornstarch-water mixture, you rapidly compact the adjacent grains, creating a solid-like structure underneath your feet.
Chapter 2: The Mechanics of Running on Oobleck
The first video titled "What Kind of Liquid Lets You Run Across Its Surface? | Street Science" demonstrates the incredible ability to run on Oobleck, showcasing the science behind this phenomenon.
When vehicles encounter a traffic bottleneck, they must navigate the situation collectively. Sudden changes in movement can lead to inefficiencies and congestion, similar to how cornstarch grains behave when a person jumps. The immediate aftermath of a jump causes the grains to compact, generating enough force to support the weight of the person.
However, once the jump concludes, the grains gradually revert to their original positions, much like how traffic flows return to normal after a jam is cleared. This underlying principle of congestion manifests in both cornstarch and traffic scenarios, despite the differences between grains of cornstarch and human drivers—it's a fascinating correlation!
If you’re intrigued by this subject, check out the video titled "Running on a Liquid Surface | Maxed Out Experiments | Science Max," which further explores the nature of Oobleck and its fascinating properties.
The 2012 study also indicated that this effect is more pronounced in shallower mixtures, where a solid core forms quickly to support weight. Conversely, in deeper pools, running becomes significantly more challenging. Keep this in mind for your next cornstarch pool experiment!
If you enjoyed this exploration of emergent phenomena, consider following the Emergent Phenomena publication, where we delve into the complexities of everyday occurrences—from materials to societal behaviors.